A few elements all metals can form more than one possible charge. An iron ion with a 2 charge would mean losing 2 electrons.

Ionic Bonding And Simple Ionic Compounds
Figure PageIndex3 shows how the charge on many ions can be predicted by the location of an element on the periodic table.
. So Na is the sodium ion. Charges of Copper ions. 1s2 2s2 2p6 3s2 3p6 3d6.
10 What possible charge s can lead acquire as an ion. But there are cases in which an atom can acquire an electrical charge. Thus a carbon ion can have a charge of anywhere from -4 to 4 depending on if it loses or gains electrons.
Charge of Krypton ion. 3- 3 5 34. 1s2 2s2 2p6 3s2 3p6 4s2 3d6.
Ca 2 is the calcium ion. Although the most common oxidation states of carbon are 4 and 2 carbon is able to make ions with oxidation states of 3 1 -1 -2 and -3. The charge of an iron ion is 2.
11 What possible charge s can iron acquire as an ion. 1- 1 5 36. An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is greater or less then the number of protons in the atom.
The charge that an atom acquires when it becomes an ion is related to the structure of the periodic table. All atoms want to have their valence electron shells filled. What possible charge can iron acquire as an ion.
Charges of Germanium ions. Which ion has a charge of 2. 2- 4 6 35.
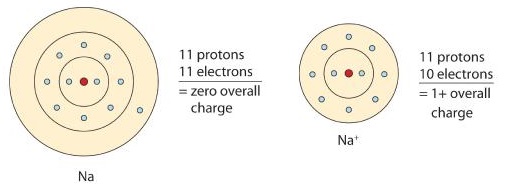
An ion is a charged atom or molecule. It is charged because the number of electrons do not equal the number of protons in the atom or molecule. The neutral sodium atom has 11 protons and 11 electrons which means it has 11 positive charges and 11 negative.
IronIII a 3 charge. That said the electron notation for a regular non-ion iron atom looks like this. Charge of Zinc ion.
An atom with a different number of electrons to protons would be called an ion. See answer 1 Best Answer. Charge of Nickel ion.
Charges of Selenium ions. Charge of Gallium ion. Copy 2 and 4 charges.
1s2 2s2 2p6 3s2 3p6 3d5. For example iron atoms can form 2 cations or 3 cations. Elements like lithium that loose their electrons form positive ions.
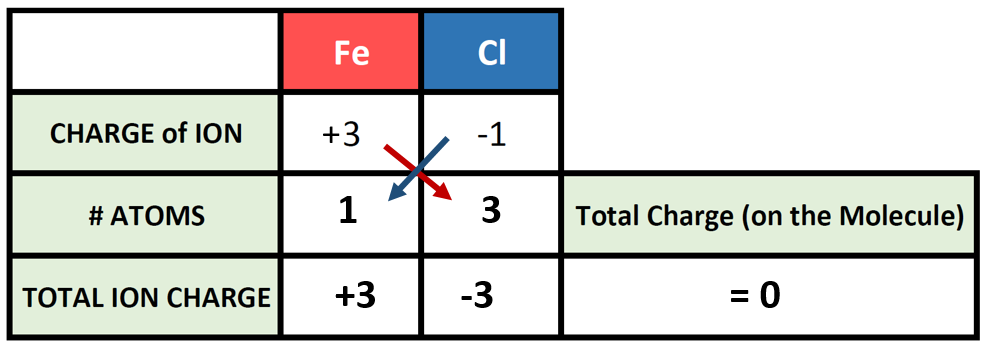
Fe3 Fe2 iron III iron II atomic 26 number ion charge ion name symbol IUPAC KEY 58 59 60 61 62 63 64 65 66 67 68 69 70 71. For example ironII has a 2 charge. Therefore the formula of Iron.
In similar fashion a 3 charge would mean losing 3 electrons. Cobalt is another element that can form more than one possible charged ion 2 and 3 while lead can form 2 or 4 cations. 13 The following questions pertain to ionic compounds of Mg².
Roman numeral notation indicates charge of ion when element commonly forms more than one ion. 4- 2 4 33. Charges of Iron ions.
Charges of Cobalt ions. 2 and 4 charges. The ironII ion has 2 charge - Fe2 The carbonate has -2 charge - CO32- the charge must be balanced so for every one ironII ion there should be one carbonate ion.
Other elements tend to gain electrons. What possible charge can lead acquire as an ion. 12 Is energy released or absorbed when neutral sodium atoms react with chlorine gas to form solid sodium chloride NaCl.
Chemistry questions and answers. Charges of Bromine ions. In order to reduce as much as possible its environmental impact inherent to industrial production Saint-Gobain PAM Canalisation supported by the French Agency for Ecological Transition ADEME has just invested nearly 10 million to acquire the largest low-carbon electric furnace in Europe for ductile iron pipes.
Without its outermost electron the lithium atom would have more positive charges 3 than negative charges -2. Symbolically we can represent this as Li 1. Charges of Arsenic ions.
An ion example For example in the compound sodium chloride table salt the sodium atom has a positive charge and the chlorine atom has a negative charge.
Ch104 Chapter 3 Ions And Ionic Compounds Chemistry
0 Comments